Engineering & Quality Solutions, Inc., is your consulting firm for expert, timely service to support your medical device projects. We offer a wide range of experience and services with particular expertise in the orthopedics industry. Our clients range from surgeon entrepreneurs with a device idea, to start-ups to multi-million dollar companies with full product offerings. We provide the extra resources you need to complete your project on-time and under budget, from idea to launch projects or single design analysis and improvement projects and everything in between. Whether it's product development, design, analysis, manufacturing, design controls and 21 CFR 820 requirements, our consultants are committed to your success.
Trust Engineering & Quality Solutions for technical expertise, excellent client service and strict confidentiality. We understand the challenges of medical device engineering, development and product support. Our goal is to help you achieve yours.
NEWS
Highlights of Products Experience
- Orthopedic Trauma Implants
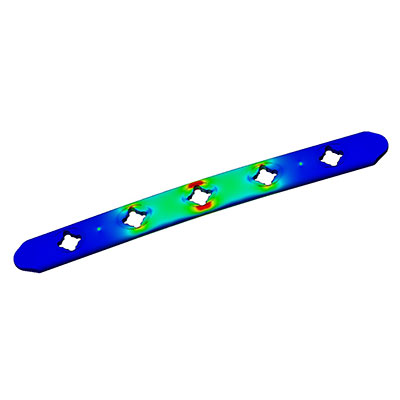
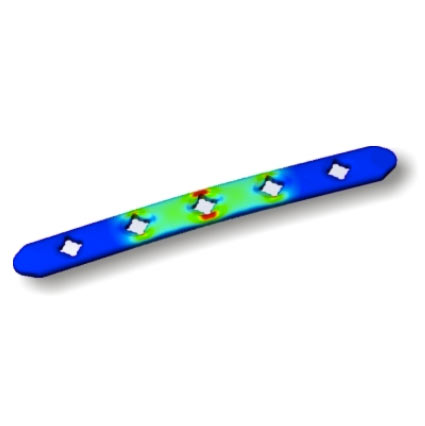
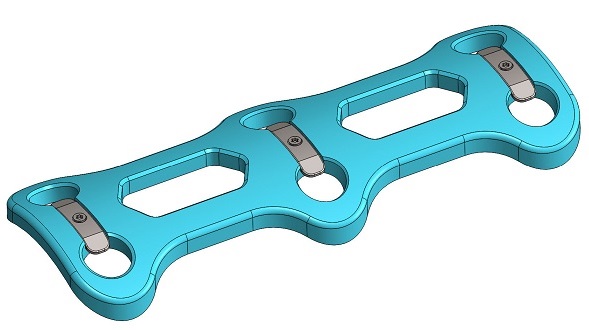
- Locking & Non-locking Plates
- IM Nails & Lag Screws
- Cortex & Cancellous Screws
- Cannulated Screws
- Locking Screws
- Orthopedic Instruments
- Hand Tools
- Drill Bits & Drill Guides
- Wires
- Spinal Surgery Instruments
- Spinal Devices
- Pedicle Screws
- Rods
- Cervical & Lumbar Plates
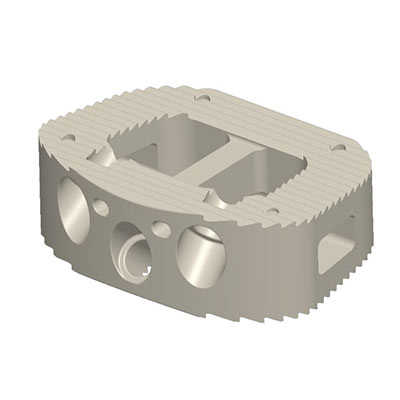
- Interbody Fusion Devices (IBFD)
- PLIF, ALIF, TLIF, DLIF
- Expandable Cages