Consulting Services
Engineering & Quality Solutions, Inc. provides a wide range of professional services to help clients meet their goals faster and easier, while never compromising on excellence. Don't hesitate to contact us to help with your projects - large or small.
FEA & ENGINEERING ANALYSIS
FEA & Engineering Analysis
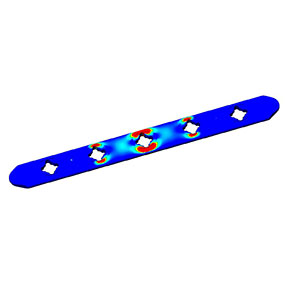
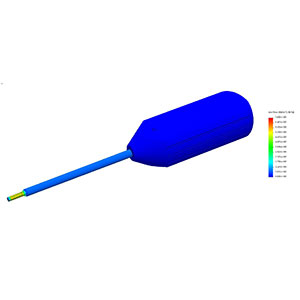
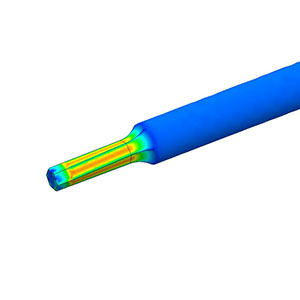
Investigation, verification or risk assessment -- Engineering & Quality Solutions, Inc., will help complete your project portfolio or resolve your design concerns. We’re experts in Finite Element Analysis (FEA) and motion simulation of orthopedic and spinal devices from single component analysis to complex assembly analysis. Our submission-ready FEA reports are professional and comply with FDA guidance for computational modeling.
FEA Slider
- Finite Element Analysis
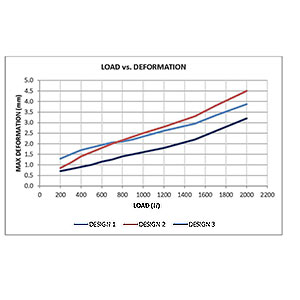
- Compare Design Iterations
- Determine Worst Case Design Versions
- Validate FEA Results to Test Lab Data
- Prepare Formal Reports for Regulatory Submissions
- Risk Management and Assessment
- Perform DFMEA & PFMEA
- Analyze Device Hazards
- Apply ISO 14971 Standard
- Design Problem Solving
- Improve Test Lab Performance
- Optimize Fit & Function
- Resolve Manufacturing & Assembly Issues
- Reduce Cost
PRODUCT DEVELOPMENT
Product Development
Engineering & Quality Solutions is your partner in full-service product development. From great idea to successful launch, our team will support yours in all phases of the design and development process. With consistent, timely tracking and communication, we’re ready to adapt and conquer any challenges your project requires to keep it on track.
PDSLider
- Design to Specifications & Requirements
- Apply ASTM & ISO Standards
- Supply Complete Drawing & Specification Packages
- Improve Fit & Function
- Resolve Manufacturing & Assembly Issues
- Reduce Cost
- Create Design Inputs & Outputs
- Conduct Design Reviews
- Complete Design Verification & Validation
- Design History File Remediation
- Plan Project Timelines
- Track Progress & Adjust to Challenges
- Product Design
- Design Problem Solving
- Design Controls & Design History Files
- Product Development Planning
FDA COMPLIANCE &; QUALITY SYSTEM
FDA Compliance & Quality System
No medical device project is complete without a compliant quality management system and proper documentation. Engineering & Quality Solutions, Inc., can set up the entire QMS or fill in a few gaps, such as compiling a robust project Design History File (DHF).
From startups to major corporations and device companies to contract manufacturers, our consultants have worked on wide range of quality system projects. We understand the regulations and standards, and we know how to apply them to various business structures to optimize compliance and efficiency.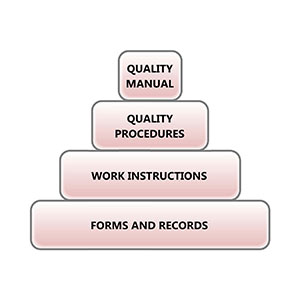
- Quality Manuals
- Internal Audits
- Procedures
- Standard Forms
- Records Management
- Design History File Remediation
- Design Inputs & Outputs
- Design Reviews
- Design Verification & Validation
- Investigations
- Corrective Actions
- FDA Reporting
- Quality System Consulting per ISO 9001, ISO 13485 & FDA 21 CFR 820
- Design Controls & Design History Files
- Product Complaint Guidance