
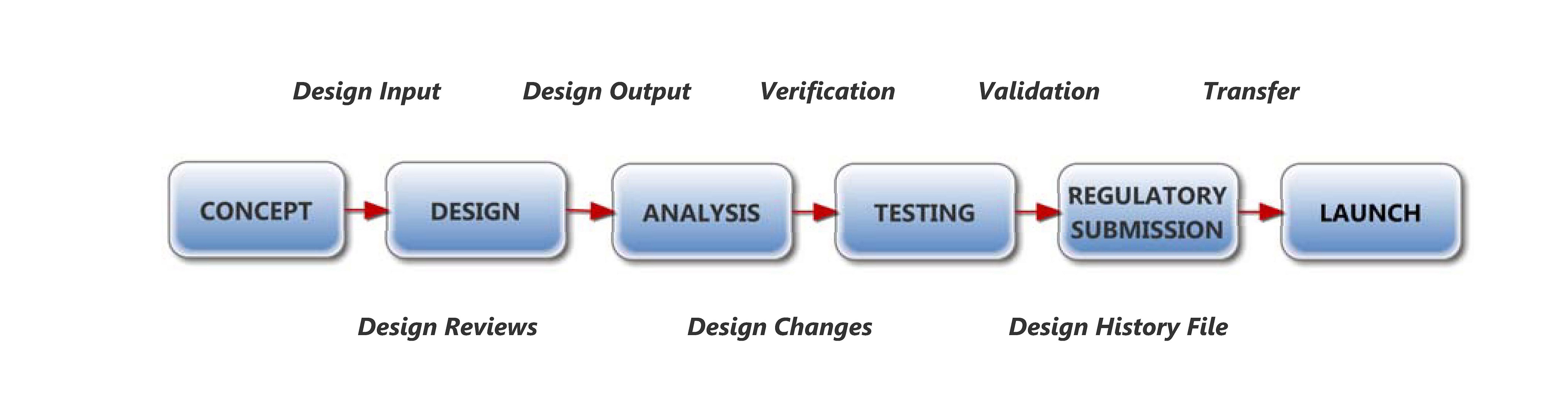
Many medical device companies find themselves uncertain of how to meet FDA design control regulations, and overwhelmed with the associated DHF workload. Lean start-ups are focused on product innovation and launch, and therefore have few resources to dedicate to DHF documentation. Established companies have legacy product DHFs that were created years ago and are in need of an update. Many companies are faced with audit findings and corrective actions related to insufficient DHF procedures and files, and they don’t know where to begin the improvement process. No matter what the scenario is, EQS can help get your DHFs and procedures in order.
EQS can ease your DHF burden by:
Contact us today to discuss your project!
Our goal is to help you meet yours.
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.
 |
 |
Consulting in: Medical Device, Orthopedic & Spinal Devices, Product Development, Design, 3D CAD,
Engineering, Finite Element Analysis, FEA, Quality Management System, QMS, ISO 13485,
ISO 9001, FDA Compliance, FDA 21 CFR 820, Design History Files & Remediation
Comments